
Now approved.
Introducing DOJOLVI <spanclass=”font-weight-normal”>(triheptanoin).
DOJOLVI® (triheptanoin) is the first and only prescription drug available in Canada indicated as a source of calories and fatty acids for the treatment of adult and pediatric patients with long-chain fatty acid oxidation disorders (LC-FAOD).
Select a type to learn more.
Select a type to learn more.
LC-FAOD=long-chain fatty acid oxidation disorders.
DOJOLVI® is publicly reimbursed in the majority of Canadian provinces
Anytime anywhere emergency access
DOJOLVI emergency storage across Canada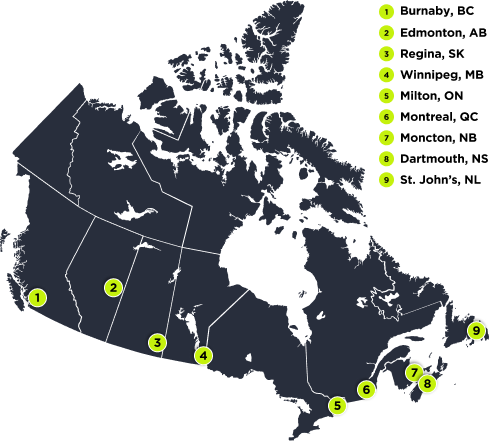
How to access DOJOLVI for emergency use for a patient with LC-FAOD
-

STEP 1
Call 1-833-388-5872 (U-LTRA) to make your request, 24 hours a day, 7 days a week
-

STEP 2
Fax completed enrolment form to 1-833-592-2273 (CARE)
Note: Visit UltraCareSupport.ca to download the UltraCare Enrolment Form -

STEP 3
Place order for DOJOLVI
Note:If purchase cannot be initiated outside of business hours, the order can still be placed. The timing of the shipment will not be affected.
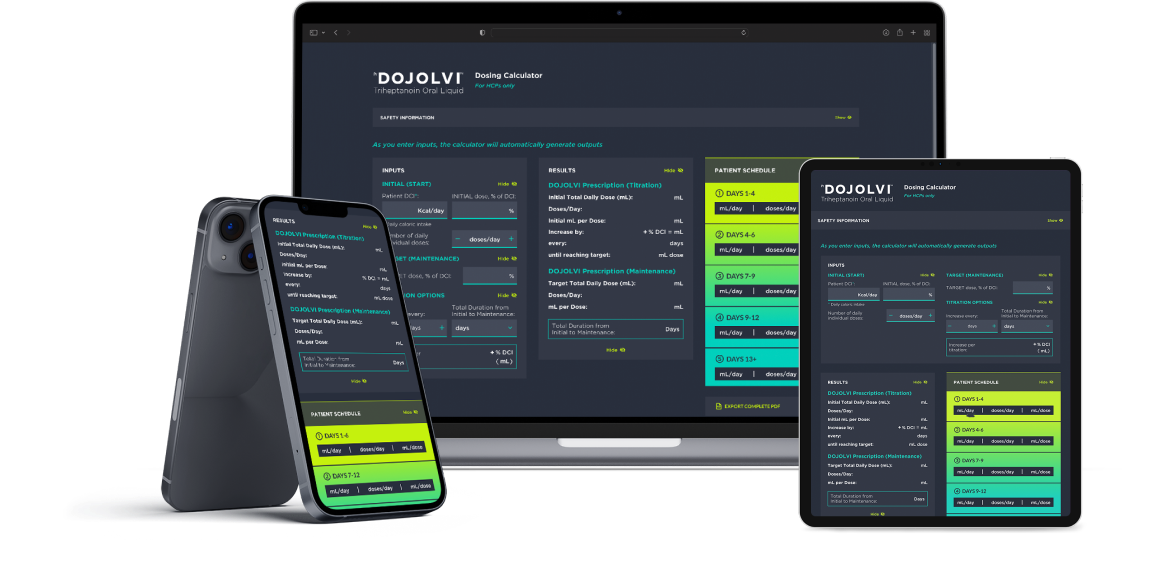
Calculate DOJOLVI
Dosing Calculator For HCPs only
This dosing calculator is designed to help you calculate the DOJOLVI daily dosing schedule for your patients.
Calculate DOJOLVI Today!Instructional Mixing Videos




Prescribe DOJOLVI
Complete the Enrolment Form with your patient and fax it to 1-833-592-2273 (CARE).
For additional support, you may also call our UltraCare Case Managers at 1-833-388-5872 (U-LTRA).
Access DOJOLVI
UltraCare® Patient Services provides a suite of services to help patients and caregivers with:
- Reimbursement Navigation
- Educational Support
- Adherence Support
- Financial Support
- Pharmacy Services
- Bilingual Support
- Reimbursement Navigation
- Educational Support
- Adherence Support
- Financial Support
- Pharmacy Services
- Bilingual Support
LC-FAOD Defined
LC-FAOD are a group of rare, often severe, and life-threatening autosomal recessive disorders that result from defective enzymes involved in the mitochondrial transport and catabolism of long-chain fatty acids (LCFAs).1-4
Each type of LC-FAOD is named for the specific enzyme that is affected.4
Select a type to learn more.
VLCAD
(very long-chain acyl-CoA dehydrogenase) deficiency
References: 1. Knottnerus SJG, Bleeker JC, Wüst RCI, et al. Rev Endocr Metab Disord. 2018;19(1):93-106. 2. Wajner M, Amaral AU. Biosci Rep. 2015;36(1):e00281. 3. Lindner M, Hoffmann GF, Matern D. J Inherit Metab Dis. 2010;33(5):521-526. 4. Wanders RJ, Ruiter JP, IJLst L, Waterham HR, Houten SM. J Inherit Metab Dis. 2010;33(5):479-494.
DOJOLVI IS A UNIQUE, ODD-CHAIN MEDIUM-LENGTH FATTY ACID
DOJOLVI is a synthetic medium odd-chain (C7) triglyceride consisting of three odd-chain, 7-carbon-length fatty acids.
It is the first and only prescription drug available in Canada indicated as a source of calories and fatty acids for the treatment of adult and pediatric patients with LC-FAOD.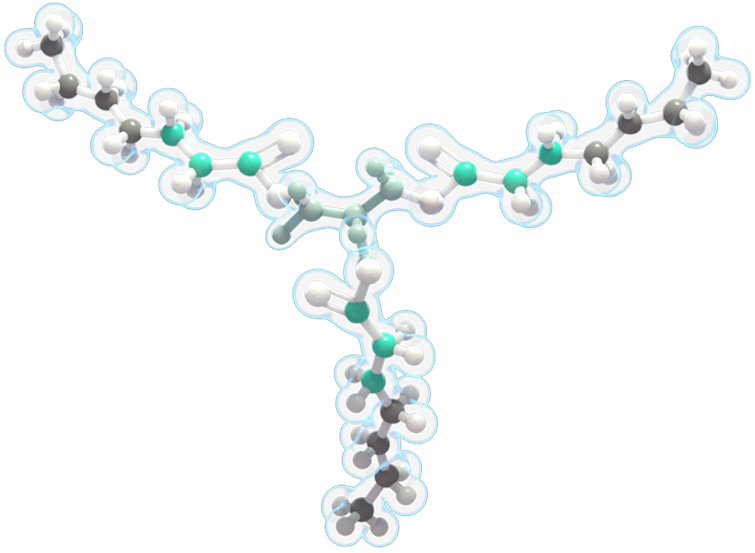
DOJOLVI is a synthetic medium odd-chain (C7) triglyceride consisting of three odd-chain, 7-carbon-length fatty acids.
It is the first and only FDA-approved treatment for patients of all ages diagnosed with LC-FAOD.
Each 7-carbon fatty acid (heptanoate) in DOJOLVI provides a source of calories and fatty acids to bypass the enzyme deficiencies in LC-FAOD for energy production and replacement.
DOJOLVI dosing and administration
Calculating the DOJOLVI dose
The recommended target daily dosage of DOJOLVI is up to 35% of the patient’s total prescribed daily caloric intake (DCI), divided into at least 4 doses and administered at mealtimes or with snacks.*
To find the DOJOLVI dose for your patient:
1
2
3
4
MULTIPLY the total DCI (in kcal) by the target percentage of the DCI that will be provided by DOJOLVI
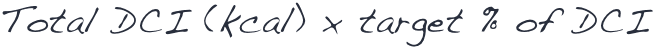
DIVIDE by 8.3 kcal/mL, the caloric value of DOJOLVI

ROUND the calculated total daily dosage of DOJOLVI (in mL) to the nearest whole number

DIVIDE the total daily dosage into at least 4 approximately equal doses to be given at each interval mixed thoroughly with a meal or snack
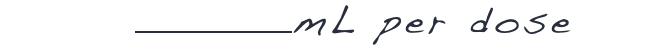
In order to reach a target daily dosage, patients may require an increase in their total fat intake. All patients treated with DOJOLVI should be under the care of a clinical specialist knowledgeable in appropriate disease-related dietary management based upon current nutritional recommendations.
The neonatal population may require higher fat intake and therefore an increased amount of DOJOLVI. Current nutritional recommendations should be considered when dosing the neonatal population.
Initiating and titrating DOJOLVI
For patients not currently taking a medium-chain triglyceride (MCT) product:
For patients switching from another MCT product:
If a patient has difficulty tolerating 1/4 of the total daily dosage at one time, more frequent, smaller doses may be considered.
Monitor patients’ total caloric intake during dosage titration, especially in patients with gastrointestinal adverse reactions, and adjust all components of the diet as needed.
If a patient experiences gastrointestinal adverse reaction(s), consider dosage reduction until the gastrointestinal symptoms resolve. Maintain the patient at the maximum tolerated dosage up to 35% DCI.
Administering DOJOLVI
Administer DOJOLVI at least 4 times per day orally or via an oral or enteral feeding tube made of silicone or polyurethane.
Use an oral syringe or measuring cup made of compatible materials to withdraw the prescribed volume of DOJOLVI from the bottle
- Compatible materials include stainless steel, glass, high-density polyethylene (HDPE), polypropylene, low-density polyethylene (LDPE), polyurethane, and silicone
- Do not prepare or administer DOJOLVI using containers, dosing syringes, or measuring cups made of polystyrene or polyvinyl chloride (PVC) plastics
- Regularly monitor the containers, dosing syringe or measuring cups that are in contact with DOJOLVI to ensure proper functioning and integrity
Add the prescribed amount of DOJOLVI to a clean bowl, cup or container, made of compatible materials as listed above, which contains an appropriate amount of semisolid food or liquid that takes into consideration the age, size and average consumption of the patient in order to ensure administration of the full dose.
If taken orally, the mixture may be stored for up to 24 hours in the refrigerator
If a dose (one of the portions taken throughout the day) is missed, take the next dose as soon as possible. Skip the missed dose if it will not be possible to take all doses in a day.
Feeding tube performance and functionality can degrade over time depending on usage and environmental conditions. In clinical trials, feeding tube dysfunction was reported in patients receiving triheptanoin.
Avoid administration of DOJOLVI in patients with pancreatic insufficiency.
For complete information on the preparation and administration of DOJOLVI, please refer to Section 3.3 of the full Product Monograph.
Instructional Dosing Videos
 Precautions
Precautions Press-in Bottle Adapter
Press-in Bottle Adapter Syringe Dosing
Syringe Dosing Syringe Cleaning
Syringe Cleaning Dosing Cup
Dosing Cup Dosing Cup Cleaning
Dosing Cup Cleaningstoring dojolvi
DOJOLVI is a clear, colourless to light yellow liquid supplied in 500 mL bottles containing 100% w/w of triheptanoin active ingredient.
Store DOJOLVI upright at room temperature between 15° to 30°C
Opened bottles of DOJOLVI can be used within 9 months or by the expiration date on the bottle, whichever is earlier
Do not dose or store DOJOLVI in containers made of polystyrene or polyvinyl chloride (PVC)

dosing guide

Download the Guide
